Class 9 Items: Drugs, Chemicals and Biological Stains Sulfa Drugs Miscellaneous Medical Equipment
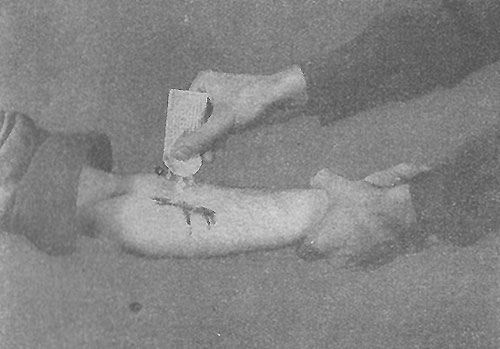
Illustration showing the application of Crystalline Sulfanilamide in an open wound on the forearm. The illustration is taken from FM 21-11 “First Aid for Soldiers”
Background:
The term “Sulfa Drugs” refers to any drug which is derived from the sulfonamide-based group. Sulfa Drugs are synthetic antimicrobial agents that contain the sulfonamide group which help the body combat infection by inhibiting the growth and multiplication of pathogenic bacteria. In 1935, the first ever sulfa drug was developed by a German scientists named Gerhard Domagk, who worked at the Bayer Laboratories, and given the trade name of Protosil. The drugs were further developed by French researchers in the late 1930s, and by exploiting the German discovery, they in fact opened the way for bacterial chemotherapy! The pioneering work carried out at these German laboratories soon became global news, as the discovery of the sulfonamide group opened a new era in medicine. Both the “John Hopkins Medical School” and the “Medical Field Service School” became involved, and after intensive research, it was decided in 1939 to introduce the antimicrobial drug for official Army use. Indeed it was not until the late 1940s and early 1950s that Sulfa Drugs began to be phased out in favor of the new Penicillin (discovered in 1928, and mass-produced in the early 1940s).

Gerhard Domagk, c. 1960.
Sulfa in the Military:
The US Army adopted two Sulfa Drugs; Crystalline Sulfanilamide and Sulfadiazine Tablets. Indeed, each soldier carried an individual First Aid Packet, containing a field dressing, and sulfa powder or tablets. These two drugs were designed to be used by both combat medics and regular soldiers in the frontline to prevent infection of severe wounds. A number of contractors were employed by the US Armed Forces to produce Sulfa Drugs during the Second World War; they were as follows:
- The Upjohn Company, Kalamazoo, Michigan
- Lederle Laboratories Inc., New York, New York
- The Anacin Manufacturing Company, Knoxville, Tennessee
- Hynson, Westcott & Dunning, Inc., Baltimore, Maryland
- E. R. Squibb & Sons, New York, New York
- Merck & Co., Inc., Rahway, New Jersey
Sulfanilamide:
Crystalline Sulfanilamide was one of the first Sulfa Drugs to be extensively used. It was designed to be sprinkled over any severe open wound before applying a sterile dressing, in an effort to prevent infection to the area. It was considered that 5 grams was a sufficient dosage for an open wound, and as a result all of the packages supplied to the Army contained no more than 5 grams of this Crystalline powder. Introduction took place around end of 1941.

Illustration showing the contents of a First Aid Packet. Of special interest is both the early and late types of Sulfa Shaker packets.
A number of different versions of packages were produced, each depending upon their manufacturer. Upjohn had adopted the package designed by William F. Irrgang, which had been developed with quick access in mind (Pat. # 1972995). Other packages containing Crystalline Sulfanilamide from the period were not accepted for patent registration until after the conflict, most notably Hynson, Westcott & Dunning’s red and yellow package found in Individual “Carlisle” Dressings. It should be noted that in 1941 a package containing 12 Sulfanilamide Tablets was also introduced.

Larger Sterile Sulfa Shaker packets, as produced by Hynson, Westcott & Dunning, Inc., and The Upjohn Company. The packet shown on the right is patented as #1972995
In addition to these individual packets of Crystalline Sulfanilamide, the military also contracted firms such as The Upjohn Company, and Hynson, Westcott & Dunning, Inc. to produce larger quantity containers. A number of boxes were supplied to the Medical Department, containing up to twelve shaker packets.

Larger boxes were supplied to Aid Stations and also in medical kits. Here, we see master boxes produced by The Upjohn Company; Hynson, Westcott and Dunning, Inc.; and E. R. Squibb & Sons. The latter example is courtesy of Martyn Bateman.
The US Army realized the need to deliver fast preventive measures against infection to battlefield casualties, and therefore prescribed that every man should carry with him a 5 gram package of Crystalline Sulfanilamide. This was ordinarily contained within the man’s First Aid Packet. However, as the war progressed the policy to sprinkle Sulfanilamide in all fresh wounds was recognized as useless, if not actually harmful, and it was gradually abandoned.
End of July 1944, the First US Army Surgeon recommended abandonment of the practice of sprinkling Sulfa powder on open fresh wounds as an anti-infection precaution; combined with the taking of Sulfa pills, this treatment resulted in excessive doses, and made wounds generally dirtier without reaching the deepest portions most in need of prophylaxis!
The discovery and development of Penicillin during the Second World War saw the phasing out of Sulfanilamide as a means of fighting infections in new, or lacerated wounds, and in many cases Penicillin (more potent and less toxic) was administered at the soonest possible time.
Sulfadiazine:
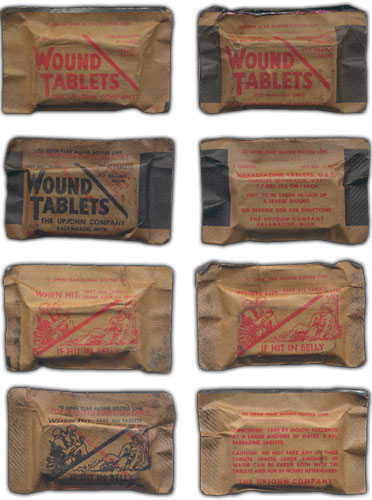
Illustration showing the various styles and designs of Sulfadiazine Tablets. All items shown have the Medical Item No. 9120400 designation. Wrappers printed in black are for use in training only. Some of the items above are courtesy of Johan Willaert
In 1942, in addition to their 5 grams of Crystalline Sulfanilamide, individual troops were also issued with 8 Tablets of Sulfadiazine (packed separately, and not included in the metal or plastic individual first aid packet). When wounded, unless in the abdomen or throat, troops were instructed to take 4 grams of Sulfadiazine orally, which amounted to 8 Tablets, with a large amount of water. The administration of Sulfadiazine would continue until surgery was initiated (where necessary). The Army prescribed the following dosages for Sulfadiazine Tablets:
- Initial Dosage: 2 to 4 grams
- Maintenance Dosage: 1 gram every 4 to 6 hours, until the drug is discontinued
Sulfadiazine is a sulfonamide antibiotic. It eliminates bacteria that cause infections by stopping the production of folic acid inside the bacterial cell, and is commonly used to treat urinary tract infections. This drug was one of the least toxic ones, and indeed the most useful of all Sulfonamides. Its use for treatment of various injuries was however discontinued after the war, becoming dependent upon the discretion of the treating medical Officer.

Illustration showing the plastic container which was wrapped inside the packages above. As can be seen the plastic container holds 8 tablets, which were to be taken all at once.
Sulfadiazine tablets were supplied in small, plastic containers, ordinarily each containing 8 Tablets (4 grams). Unlike Crystalline Sulfanilamide, the practice of administrating Sulfadiazine continued long into the war, and indeed limited supplies of Sulfadiazine meant that Prisoners of War received only Sulfadiazine Tablets as a means of treating minor or mild wounds.
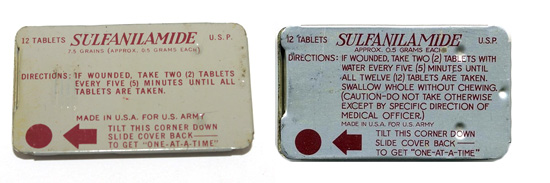
Illustration showing two variants of the small metal tin containing 12 Sulfanilamide tablets, patented by the Brisbane Box Corporation, Detroit, Michigan and manufactured by Lederle. Little information exists regarding these items, except that they were introduced in late 1941 as item # 91212, with limited distribution, and eventually replaced in 1942 by cheaper packing material (due to war restrictions on metal). They were therefore replaced by wrappers made of aluminum foil protected by Kraft tarpaper with reduced contents, i.e. only 8 tablets.
It must be noted that liberal use of Sulfa Drugs and Penicillin in WW2 kept the incidence of serious wound infection low, in spite of surgical backlog, and the fact that many casualties occurred on pastures and farmland contaminated with animal and human feces.
Remark:
Other products of the Sulfonamide family were Sulfamerizine, Sulfaguanidine, Sulfapyridine, and Sulfathiazole.
Caution:
Almost every packet of Sulfadiazine Tablets supplied to the United States Army indicated that the contents should not be administered if the casualty was hit in the stomach. This warning was issued due to the drug’s ability to cause a sense of nausea and abdominal cramps, and administering the drug in these situations could only further enhance these side effects. Apart from this it was later found that necessary precaution was to be taken when employing compounds of the Sulfonamide group, because of a possible sensitivity of the patient, resulting in toxic reactions to the kind of drug administered.

Larger (24 Tablet) Sulfadiazine container. Boxes such as this would have been supplied primarily in First Aid Kits. Both examples are manufactured by E. R. Squibb & Sons, although the bottom one is distributed under licence by Conray Products Co. Both examples above are from the collection of Martyn Bateman.
